Randomization and Trial Supply Management (RTSM) Market Overview: Key Drivers and Challenges 2022–2029
Randomization and Trial Supply Management (RTSM) Market Overview: Key Drivers and Challenges 2022–2029
Blog Article
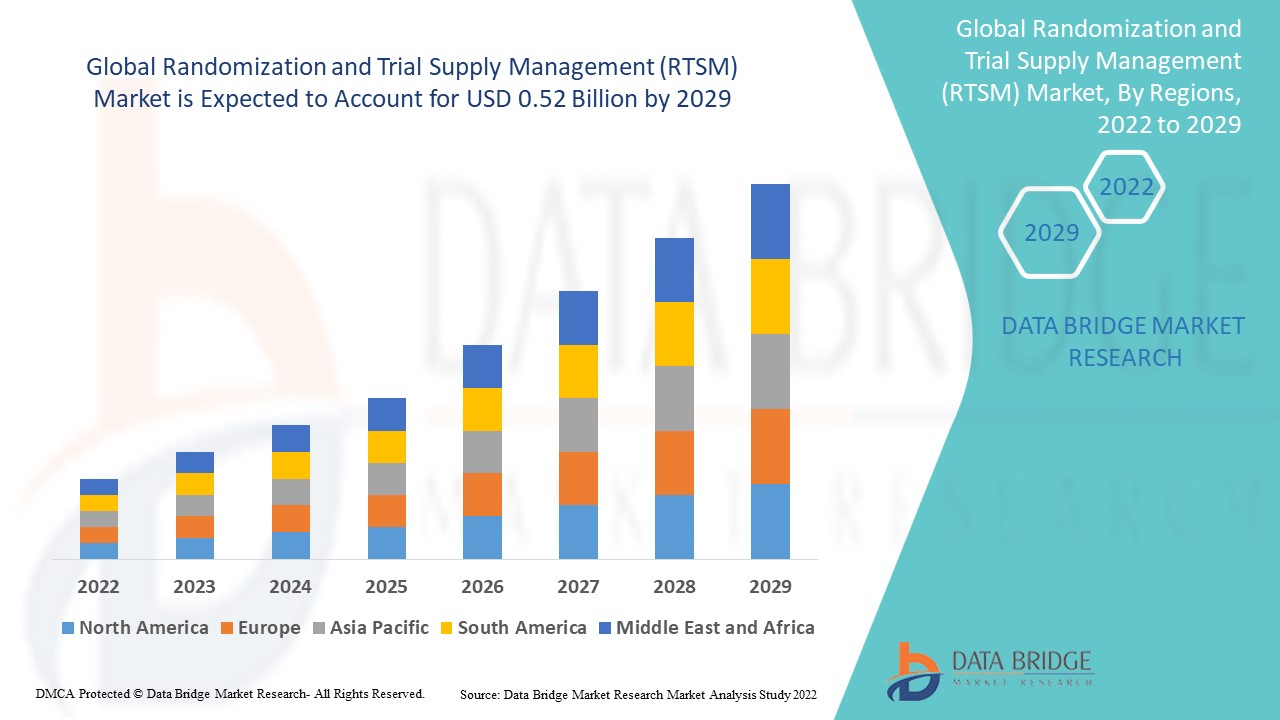
The Randomization and Trial Supply Management (RTSM) Market sector is undergoing rapid transformation, with significant growth and innovations expected by 2029. In-depth market research offers a thorough analysis of market size, share, and emerging trends, providing essential insights into its expansion potential. The report explores market segmentation and definitions, emphasizing key components and growth drivers. Through the use of SWOT and PESTEL analyses, it evaluates the sector’s strengths, weaknesses, opportunities, and threats, while considering political, economic, social, technological, environmental, and legal influences. Expert evaluations of competitor strategies and recent developments shed light on geographical trends and forecast the market’s future direction, creating a solid framework for strategic planning and investment decisions.
Brief Overview of the Randomization and Trial Supply Management (RTSM) Market:
The global Randomization and Trial Supply Management (RTSM) Market is expected to experience substantial growth between 2024 and 2031. Starting from a steady growth rate in 2023, the market is anticipated to accelerate due to increasing strategic initiatives by key market players throughout the forecast period.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-randomization-and-trial-supply-management-rtsm-market
Which are the top companies operating in the Randomization and Trial Supply Management (RTSM) Market?
The report profiles noticeable organizations working in the water purifier showcase and the triumphant methodologies received by them. It likewise reveals insights about the share held by each organization and their contribution to the market's extension. This Global Randomization and Trial Supply Management (RTSM) Market report provides the information of the Top Companies in Randomization and Trial Supply Management (RTSM) Market in the market their business strategy, financial situation etc.
MEDICAL Information Technology Inc. (U.S), SAP (Germany), CPSI (U.S), Meta (U.S), Elinext (U.S), EPIC Systems Corporation (U.S), INFOR (U.S), Cognizant (U.S), Oracle (U.S), Jag products LLC (U.S), Allscripts Healthcare LLC (U.S), Optum Inc. (U.S), Cerner Corporation (U.S), Change Healthcare (U.S), Koninklijke Philips N.V. (Netherlands), athenahealth (U.S), eClinicalWorks (U.S)
Report Scope and Market Segmentation
Which are the driving factors of the Randomization and Trial Supply Management (RTSM) Market?
The driving factors of the Randomization and Trial Supply Management (RTSM) Market are multifaceted and crucial for its growth and development. Technological advancements play a significant role by enhancing product efficiency, reducing costs, and introducing innovative features that cater to evolving consumer demands. Rising consumer interest and demand for keyword-related products and services further fuel market expansion. Favorable economic conditions, including increased disposable incomes, enable higher consumer spending, which benefits the market. Supportive regulatory environments, with policies that provide incentives and subsidies, also encourage growth, while globalization opens new opportunities by expanding market reach and international trade.
Randomization and Trial Supply Management (RTSM) Market - Competitive and Segmentation Analysis:
**Segments**
- Based on type, the global Randomization and Trial Supply Management (RTSM) market is segmented into web-hosted (on-demand) RTSM, licensed enterprise (on-premise) RTSM.
- By product, the market is categorized into software and services.
- On the basis of delivery mode, the market is divided into onsite and cloud-based.
- Considering the end-user segmentation, the market is classified into pharmaceutical and biopharmaceutical companies, contract research organizations, and others.
As we move towards 2029, the Randomization and Trial Supply Management (RTSM) market is expected to witness significant growth due to the increasing demand for advanced technologies in clinical trials to enhance the overall efficiency and accuracy of the trial processes. The web-hosted (on-demand) RTSM segment is likely to dominate the market as it provides ease of access, flexibility, and cost-effectiveness to users. Additionally, the rising adoption of cloud-based delivery mode is expected to propel market growth by offering scalability, improved data security, and remote access to RTSM solutions.
**Market Players**
- Oracle
- Parexel International Corporation
- Bioclinica
- YPrime
- Sharp
- Cmed Technology Ltd.
- ICON plc
- Almac Group
- Cenduit LLC
- The Clinigen Group
These market players are anticipated to play a crucial role in shaping the competitive landscape of the Randomization and Trial Supply Management (RTSM) market in the coming years. Strategic collaborations, product innovations, and mergers and acquisitions are some of the key strategies adopted by these companies to strengthen their market position and gain a competitive edge. With a focus on offering integrated and efficient RTSM solutions, these players are expected to drive market growth and cater to the evolving needs of the pharmaceutical and biopharmaceutical companies and contract research organizations.
https://www.databridgemarketresearch.com/reports/global-randomization-and-trial-supply-management-rtsm-marketThe Randomization and Trial Supply Management (RTSM) market is poised for substantial growth in the upcoming years, driven by the escalating demand for advanced technologies in clinical trials. The adoption of web-hosted RTSM solutions is poised to surge due to the convenience, flexibility, and cost-effectiveness they offer to users. This segment is likely to dominate the market landscape, catering to the evolving needs of pharmaceutical and biopharmaceutical companies, contract research organizations, and other end-users. Moreover, the increasing preference for cloud-based delivery modes is expected to further fuel market expansion by providing enhanced scalability, superior data security measures, and remote accessibility to RTSM solutions. As the industry gears up for 2029, these trends underscore the pivotal role technology will play in optimizing the efficiency and accuracy of clinical trial processes.
In this dynamic market environment, key players such as Oracle, Parexel International Corporation, Bioclinica, YPrime, Sharp, Cmed Technology Ltd., ICON plc, Almac Group, Cenduit LLC, and The Clinigen Group are set to shape the competitive landscape through strategic initiatives. These market players are anticipated to engage in collaborations, product innovations, and strategic acquisitions to fortify their market positions and gain a competitive advantage. By focusing on delivering integrated and efficient RTSM solutions, these companies are expected to drive market growth and address the evolving requirements of the pharmaceutical and biopharmaceutical sectors along with contract research organizations. As the market evolves, these players are likely to pave the way for innovative solutions that streamline the clinical trial processes, enhance patient outcomes, and drive operational efficiencies for stakeholders across the industry.
Looking ahead, market players are expected to leverage cutting-edge technologies, data-driven insights, and customer-centric approaches to differentiate their offerings and drive sustainable growth. By tapping into emerging trends, such as the increasing emphasis on real-time data analytics, personalized medicine, and patient-centric trial designs, these companies can stay ahead of the curve and meet the evolving demands of the healthcare and life**Market Players**
- MEDICAL Information Technology Inc. (U.S)
- SAP (Germany)
- CPSI (U.S)
- Meta (U.S)
- Elinext (U.S)
- EPIC Systems Corporation (U.S)
- INFOR (U.S)
- Cognizant (U.S)
- Oracle (U.S)
- Jag products LLC (U.S)
- Allscripts Healthcare LLC (U.S)
- Optum Inc. (U.S)
- Cerner Corporation (U.S)
- Change Healthcare (U.S)
- Koninklijke Philips N.V. (Netherlands)
- athenahealth (U.S)
- eClinicalWorks (U.S)
In the rapidly evolving landscape of Medical Information Technology, key players such as MEDICAL Information Technology Inc., SAP, CPSI, Meta, Elinext, EPIC Systems Corporation, INFOR, Cognizant, Oracle, Jag products LLC, Allscripts Healthcare LLC, Optum Inc., Cerner Corporation, Change Healthcare, Koninklijke Philips N.V., athenahealth, and eClinicalWorks play a pivotal role in driving innovation, efficiency, and effectiveness in healthcare IT solutions. These companies are at the forefront of leveraging cutting-edge technologies, data-driven insights, and customer-centric approaches to cater to the evolving needs of the healthcare industry and enhance patient outcomes.
As the healthcare sector continues to prioritize digital transformation and technological advancements, the demand for integrated and efficient medical information technology solutions is
North America, particularly the United States, will continue to exert significant influence that cannot be overlooked. Any shifts in the United States could impact the development trajectory of the Randomization and Trial Supply Management (RTSM) Market. The North American market is poised for substantial growth over the forecast period. The region benefits from widespread adoption of advanced technologies and the presence of major industry players, creating abundant growth opportunities.
Similarly, Europe plays a crucial role in the global Randomization and Trial Supply Management (RTSM) Market, expected to exhibit impressive growth in CAGR from 2024 to 2029.
Explore Further Details about This Research Randomization and Trial Supply Management (RTSM) Market Report https://www.databridgemarketresearch.com/reports/global-randomization-and-trial-supply-management-rtsm-market
Key Benefits for Industry Participants and Stakeholders: –
- Industry drivers, trends, restraints, and opportunities are covered in the study.
- Neutral perspective on the Randomization and Trial Supply Management (RTSM) Market scenario
- Recent industry growth and new developments
- Competitive landscape and strategies of key companies
- The Historical, current, and estimated Randomization and Trial Supply Management (RTSM) Market size in terms of value and size
- In-depth, comprehensive analysis and forecasting of the Randomization and Trial Supply Management (RTSM) Market
Geographically, the detailed analysis of consumption, revenue, market share and growth rate, historical data and forecast (2024-2031) of the following regions are covered in Chapters
The countries covered in the Randomization and Trial Supply Management (RTSM) Market report are U.S., copyright and Mexico in North America, Brazil, Argentina and Rest of South America as part of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA
Detailed TOC of Randomization and Trial Supply Management (RTSM) Market Insights and Forecast to 2029
Part 01: Executive Summary
Part 02: Scope Of The Report
Part 03: Research Methodology
Part 04: Randomization and Trial Supply Management (RTSM) Market Landscape
Part 05: Pipeline Analysis
Part 06: Randomization and Trial Supply Management (RTSM) Market Sizing
Part 07: Five Forces Analysis
Part 08: Randomization and Trial Supply Management (RTSM) Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers And Challenges
Part 13: Randomization and Trial Supply Management (RTSM) Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Browse More Reports:
Japan: https://www.databridgemarketresearch.com/jp/reports/global-randomization-and-trial-supply-management-rtsm-market
China: https://www.databridgemarketresearch.com/zh/reports/global-randomization-and-trial-supply-management-rtsm-market
Arabic: https://www.databridgemarketresearch.com/ar/reports/global-randomization-and-trial-supply-management-rtsm-market
Portuguese: https://www.databridgemarketresearch.com/pt/reports/global-randomization-and-trial-supply-management-rtsm-market
German: https://www.databridgemarketresearch.com/de/reports/global-randomization-and-trial-supply-management-rtsm-market
French: https://www.databridgemarketresearch.com/fr/reports/global-randomization-and-trial-supply-management-rtsm-market
Spanish: https://www.databridgemarketresearch.com/es/reports/global-randomization-and-trial-supply-management-rtsm-market
Korean: https://www.databridgemarketresearch.com/ko/reports/global-randomization-and-trial-supply-management-rtsm-market
Russian: https://www.databridgemarketresearch.com/ru/reports/global-randomization-and-trial-supply-management-rtsm-market
Data Bridge Market Research:
Today's trends are a great way to predict future events!
Data Bridge Market Research is a market research and consulting company that stands out for its innovative and distinctive approach, as well as its unmatched resilience and integrated methods. We are dedicated to identifying the best market opportunities, and providing insightful information that will help your business thrive in the marketplace. Data Bridge offers tailored solutions to complex business challenges. This facilitates a smooth decision-making process. Data Bridge was founded in Pune in 2015. It is the product of deep wisdom and experience.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1299
Email:- corporatesales@databridgemarketresearch.com Report this page